
Glutamate is best known for its role a a neurotransmitter – a brain signaling molecule that nerve cells (neurons) use to communicate to other neurons. So, today let’s look at Glutamate (Glu, E)! And we can get a better appreciation and understanding of proteins if we look at those letters. Those generic parts are attached to a central “alpha carbon” (Ca), which is also attached to one of 20 unique side chains (“R groups”) which have different properties (big, small, hydrophilic (water-loving), hydrophobic (water-avoided), etc.) & proteins have different combos of them, so the proteins have different properties.
#Do hydrophobic amino acids form ionic bonds free#
The reason for the “2 options” in parentheses is that these groups’ protonation state (how many protons (H⁺ ) they have) depends on the pH (which is a measure of how many free H⁺ are around to take).⠀ More on amino acids in general here but the basic overview is: amino acids have generic “amino” (NH₃⁺/NH₂) & “carboxyl” (COOH/COO⁻) groups that let them link up together through peptide bonds (N links to C, H₂O lost, and the remaining “residual” parts are called residues). Each day I’m going to bring you the story of one of these “charms” – what we know about it and how we know about it, where it comes from, where it goes, and outstanding questions nobody knows. There are 20 (common) genetically-specified ones, each with a generic backbone with to allow for linking up through peptide bonds to form chains (polypeptides) that fold up into functional proteins, as well as unique side chains (aka “R groups” that stick off like charms from a charm bracelet). Amino acids are the building blocks of proteins. It’s Day 19 of #20DaysOfAminoAcids – the bumbling biochemist’s version of an advent calendar. Alternatively, a more ordered and repetitive tertiary structure invariably results in a structural protein such as collagen.MSG? Fine by me! It’s just a salt of glutamate, so why does everyone gotta hate? From giving food its rich umami taste, to helping your cells safely remove nitrogenous waste, there’s a lot to love about this protein letter, so today I want to help you get to know it better! It’s more “popular” for its role as a brain messenger, but for those functions to brain experts I shall defer – instead, I hope you won’t become irate if I focus on some of the other (just as, if not more so) vital functions of glutamate (Glu, E)!

The resultant structure may be globular with a myriad of possible functions including enzyme catalysis, membrane transport, reception of molecules or hormonal control. It is this total entropic drive along with the bonds from transient dipoles (van der Waals') in the core which shapes a protein's 3D conformation. In an aqueous medium, hydrophobic amino acids will tend to congregate in the sheltered core of the structure while hydrophilic amino acids will associate with the water on the exterior.

Despite each bond listed being indivually quite strong, it is the cumulative contribution of the hydrophobic effect and van der Waals' forces that exerts the key influence on tertiary structure. Due to the typical reducing environment within cells, it is more common to find disulfide bridges within poteins in the hostile extracellular environment such as with the peptide hormone insulin. Disulfide bridges are strong covalent bonds which form exclusively between cysteine residues and may unite distant regions of the polypeptide chain. However, it must be noted that these are quite rare and much more heavily involved in the quaternary sturcture.
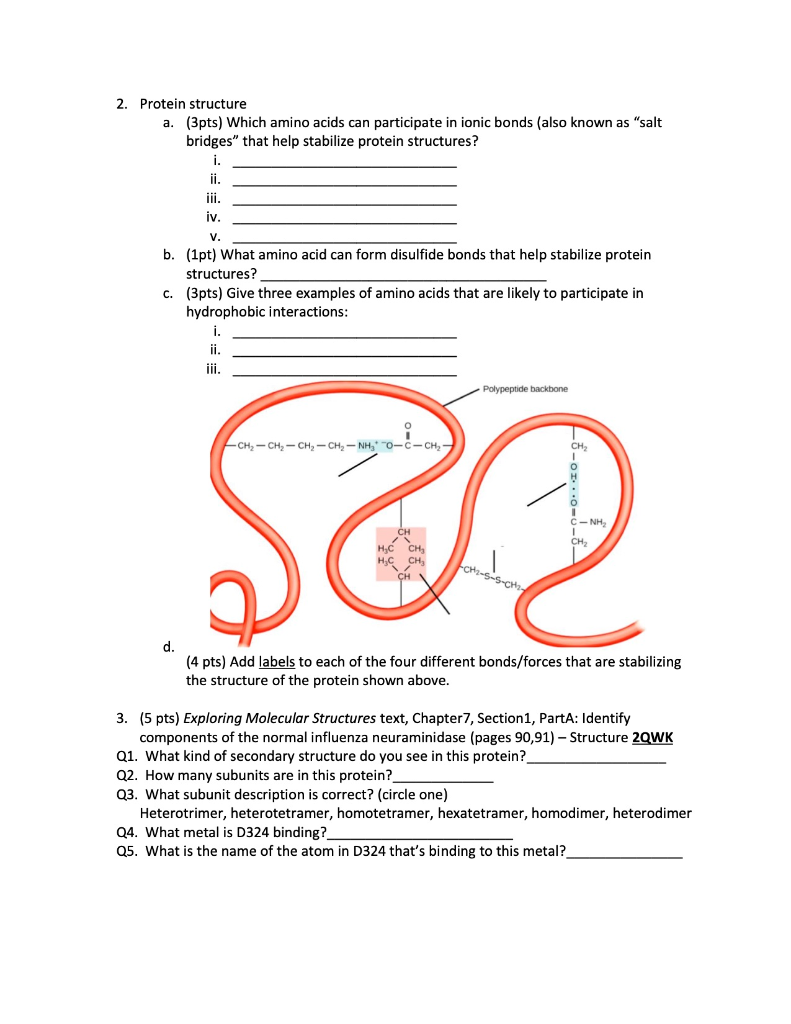
Ionic bonds (salt bridges) may also exist between basic and acidic side chains of amino acids (e.g. Firstly, hydrogen bonds further to those in the secondary structure may form between the R-groups of polar amino acids such as serine and aspartate. Bonds in a protein's tertiary structure occur entirely between the highly variable R-groups of the amino acyl residues.


 0 kommentar(er)
0 kommentar(er)
